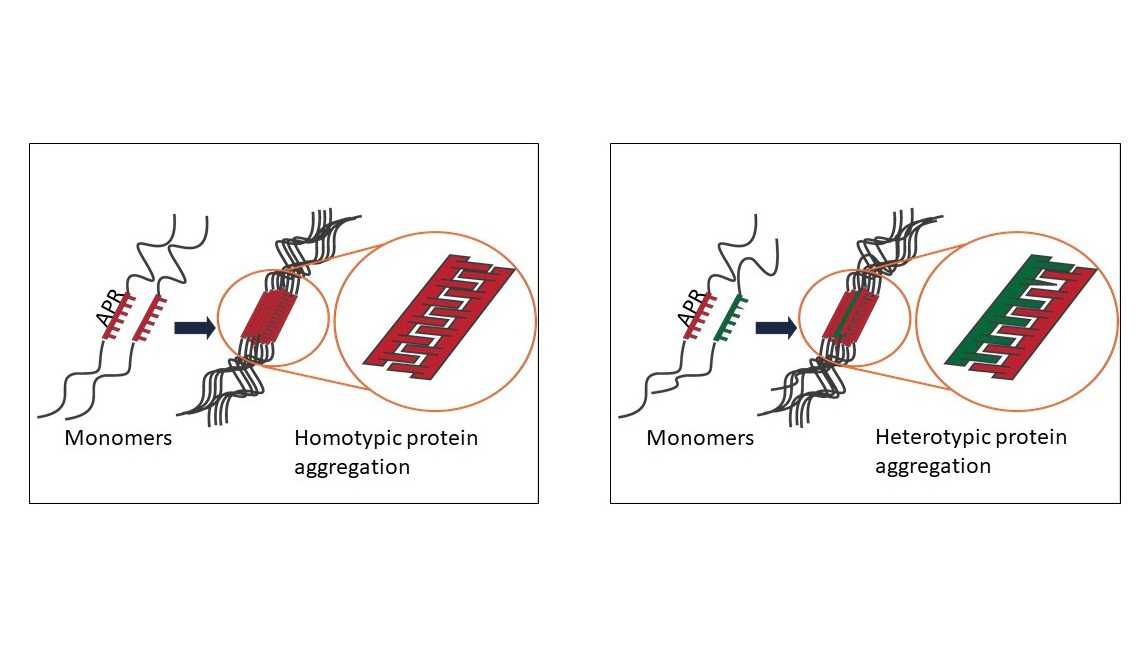
Heterotypic Protein Aggregation
As explained in the section about aggregation-prone regions (APRs), the self-assembly of APRs can drive protein aggregation.
Because of the structural restraints of the “cross-beta" conformation, amyloid assembly is a very specific process: even a single point mutation can prevent aggregation in some cases (e.g., the ‘species barriers’ observed in prions). For this reason, amyloid aggregation has been primarily considered a homotypic interaction, i.e., an interaction between identical segments of proteins.
However, interactions between amyloids and other proteins do occur, and such heterotypic interactions are increasingly observed as modifiers of amyloid assembly in disease-associated and functional amyloids [1]. As the example of the RIPK1-RIPK3 necrosome signalling complex showed, two proteins that contain very similar APRs can form a heterotypic amyloid aggregate that has an amyloid core similar to that of self-aggregating amyloids.
Heterotypic amyloid interactions are compatible with the strain hypothesis because the concept that a protein can form morphologically distinct aggregates (strains) that follow the structural characteristics of the “seeding” molecule holds true in cases of hetero-assembly, as well.
Our laboratory has shown that heterotypic interactions can form if the interacting fragments are structurally compatible with the cross-beta structure [2]. We also showed that such interactions can modify the formation of amyloid fibres by amyloid-beta [3] or tau [4].
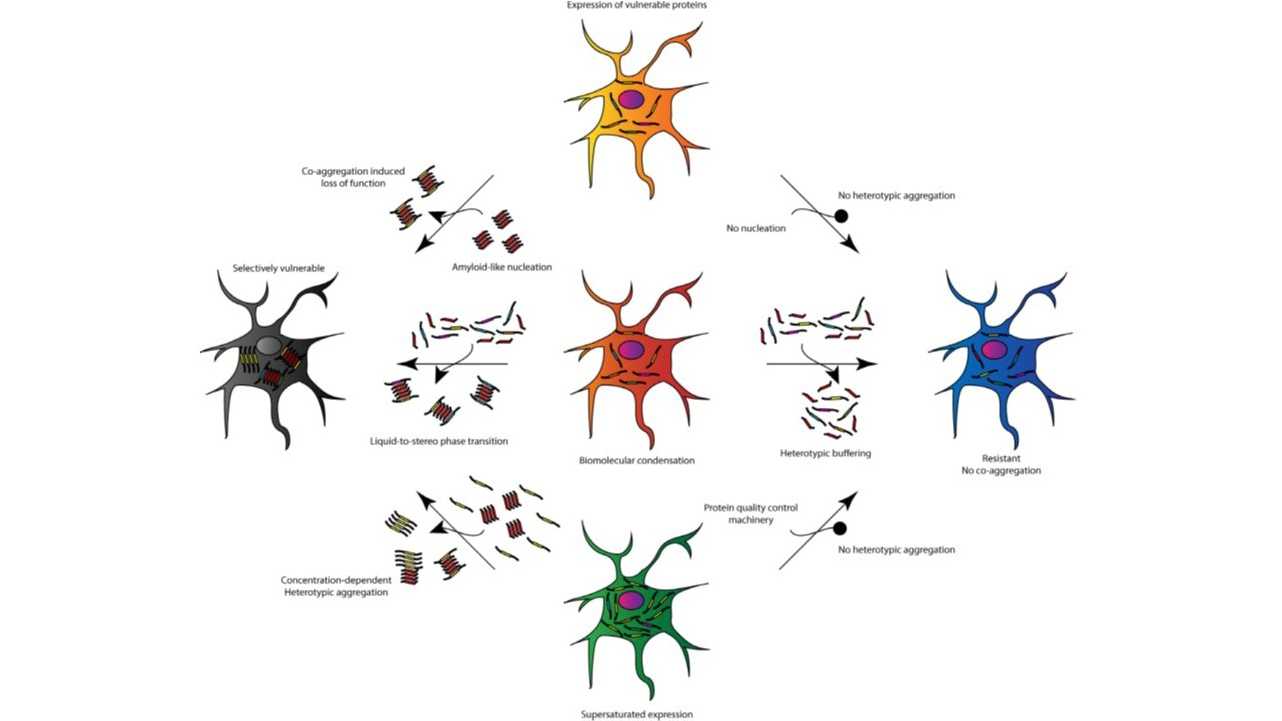
What makes heterotypic amyloid interactions exciting is that they might explain phenomena like protein co-deposition in amyloid plaques, polymorphism, transmissibility and prion-like behaviour! A recent validation of the in vivo importance of heterotypic amyloid interactions came from a collaboration with the Neher and Jucker labs. Our joint publication shows that the most common human amyloid protein (medin, also known as lactahedrin) directly interacts and co-aggregates with amyloid-beta (Aβ), the protein that forms plaques in the brains of Alzheimer’s disease patients. Since medin amyloid fibers are present in the aortic wall of almost all people over 50 years of age, our findings make medin a prime therapeutic target to prevent vascular damage in Alzheimer’s disease (5).
We believe that heterotypic interactions of amyloid with the background proteome can also explain the selective vulnerability of specific tissues or cell populations to the deposition of amyloids. Our lab has received funding from the National Institute on Aging, the Stichting Alzheimer Onderzoek (SAO-FRA) and the Funds for Scientific Research Flanders (FWO) to investigate cell-specific protein interactions with amyloid-forming proteins in neurodegenerative diseases.
References
- Konstantoulea K, Louros N, Rousseau F, Schymkowitz J (2022) Heterotypic interactions in amyloid function and disease. FEBS J 289 (8):2025-2046. doi:10.1111/febs.15719
- Louros N, Orlando G, De Vleeschouwer M, Rousseau F, Schymkowitz J (2020) Structure-based machine-guided mapping of amyloid sequence space reveals uncharted sequence clusters with higher solubilities. Nature communications 11 (1):3314. doi:10.1038/s41467-020-17207-3
- Konstantoulea K, Guerreiro P, Ramakers M, Louros N, Aubrey LD, Houben B, Michiels E, De Vleeschouwer M, Lampi Y, Ribeiro LF, Wit J, Xue WF, Schymkowitz J, Rousseau F (2022) Heterotypic Amyloid beta interactions facilitate amyloid assembly and modify amyloid structure. 0261-4189 41 (2). doi:10.15252/embj.2021108591
- Louros N, Ramakers M, Michiels E, Konstantoulea K, Morelli C, Garcia T, Moonen N, D'Haeyer S, Goossens V, Thal DR, Audenaert D, Rousseau F, Schymkowitz J (2022) Mapping the sequence specificity of heterotypic amyloid interactions enables the identification of aggregation modifiers. Nature communications 13 (1):1351. doi:10.1038/s41467-022-28955-9
- Wagner J, Degenhardt K, Veit M, Louros N, Konstantoulea K, Skodras A, Wild K, Liu P, Obermuller U, Bansal V, Dalmia A, Hasler LM, Lambert M, De Vleeschouwer M, Davies HA, Madine J, Kronenberg-Versteeg D, Feederle R, Del Turco D, Nilsson KPR, Lashley T, Deller T, Gearing M, Walker LC, Heutink P, Rousseau F, Schymkowitz J, Jucker M, Neher JJ (2022) Medin co-aggregates with vascular amyloid-beta in Alzheimer's disease. Nature 612 (7938):123-131. doi:10.1038/s41586-022-05440-3